Abstract
Hydroxyurea Mucocutaneous Toxicity: A Prospective Cohort Study of 110 ET and PV Patients from a Single Institution. Carlos Besses1,3, Francesc Garcia-Pallarols1,3, Anna Angona1,3, Marta Bertolín2, Daniel López Castillo2, Concepción Fernández-Rodríguez3, Laura Camacho3, Lierni Fernández3, Elena Torres1,3, Raquel Longarón4,3, Antonio Salar1,3, Alberto Alvarez-Larrán1,3, Beatriz Bellosillo4,3, Ramon María Pujol2, Agustí Toll2. 1 Hematology Department, 2 Dermatology Department, 3 Group of Applied Clinical Research in Hematology, 4 Pathology Department, Hospital del Mar-IMIM, Barcelona, Spain.
Introduction. Mucocutaneous Hydroxyurea (HU)-related toxicity has been reported in 5-11% of ET and PV patients. This incidence arises from retrospective small series and multicenter studies but no information is available using a prospective systematic approach. The aim of our study was to analyze prospectively the incidence, type and cumulative incidence of cutaneous adverse events (CAE) in a cohort of ET and PV patients treated with HU in a single institution.
Methods. A total of 110 patients (74 ET, 36 PV) were included. Diagnosis of ET and PV was performed according to WHO criteria. All patients were assessed by a dermatologist at baseline (immediately before starting HU) and every 6 months until withdrawal of the drug or last follow-up. CAE were categorized as actinic keratoses (AK), leg ulcers, alopecia, hyperpigmentation of skin and nails, aphthous ulcers and mucositis, non-melanoma skin cancer (NMSC) including basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) and others. Both SCC and leg ulcers were considered as serious CAE. ELN criteria for resistance/intolerance to HU in ET and PV were applied with serious CAE being considered as unacceptable non-hematological toxicity. Baseline evaluation included assessment of previous AK and NMSC, ulcers, sun exposure and skin type among other dermatologic parameters. Hematologic values, type of gene mutation, time on HU and cause of HU discontinuation were also recorded.
Results. Baseline dermatologic evaluation showed 13 patients with AK, 9 patients with 11 NMSC (8 BCC, 3SCC), and 4 patients with leg ulcers. With a median follow-up of 43 months, 66 out of 110 patients (60%) presented CAE. Median time to first CAE was 21 months (CI 95%:11-32). First CAE consisted of aphthous ulcers and mucositis (n=20), AK (n=19), alopecia (n=12), hyperpigmentation (n=7), leg ulcers (n=4), SCC (n=2) and others (n=2). A total number of 195 CAE were observed in the 110 patients during follow-up: AK (n=103) in 32 (29%), aphthous ulcers and mucositis (n=26) in 24 (21.8%), alopecia (n=15) in 15 (13.6%), SCC (n=15) in 8 (7.3%), hyperpigmentation (n=11) in 10 (9.1%), BCC (n=9) in 9 (8.2%), leg ulcers (n=8) in 8 (7.3%) and others (n=8) in 7 (6.4%). Fourteen out of 66 patients with CAE (21%) developed serious CAE (15 SCC and 8 leg ulcers). HU discontinuation was recorded in 16 patients, being CAE the cause of discontinuation in 7 patients (5 leg ulcers and 2 SCC).
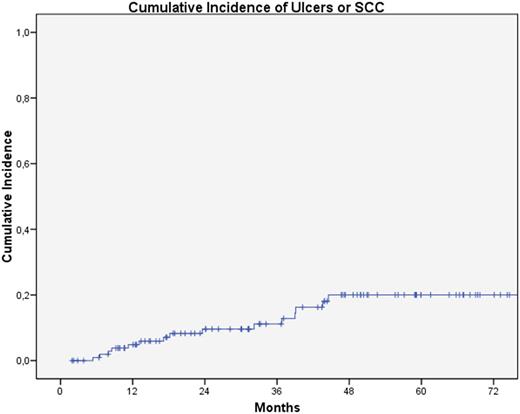
The cumulative incidence of any CAE at 12, 24, 36 and 48 months was 31.2%, 51.9%, 60.9% and 69.6%, respectively. The cumulative incidence of serious CAE (SCC and leg ulcers) at 12, 24, 36 and 48 months was 4.9%, 9.6%, 11.2% and 20%, respectively (Fig. 1). Univariate analysis showed SCC was more frequent in patients with previous AK (p = 0.026).
Conclusions. 1. Prospective and systematic dermatologic periodic examination of HU-treated PV and ET patients shows a higher incidence of CAE than classically reported with 21% of patients with CAE developing unacceptable cutaneous HU-related toxicity. 2. Clinicians should be aware of the frequency and severity of CAE induced by HU and recommend periodic dermatologic monitoring.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal